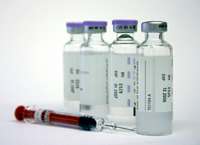
On July 23, the U.S. Court of Appeals for the District of Columbia Circuit unanimously affirmed a lower court ruling that the Food and Drug Administration (FDA) failed to fulfill its duties under the Food, Drug, and Cosmetic Act (FDCA) when it permitted without inspection the importation of foreign drugs for use in lethal injections. The Court concluded, “The FDCA imposes mandatory duties upon the agency charged with its enforcement. The FDA acted in derogation of those duties by permitting the importation of thiopental, a concededly misbranded and unapproved new drug, and by declaring that it would not in the future sample and examine foreign shipments of the drug despite knowing they may have been prepared in an unregistered establishment.” In 2009, the last U.S. manufacturer of sodium thiopental announced it would cease production of the drug commonly used by states in lethal injections. Some states then turned to international manufacturers and imported drugs which were not approved by the FDA. The ruling rejected the FDA’s claim that it had discretion to allow unapproved drugs into the U.S. Read full text of the ruling.
The Court of Appeals overturned the lower court’s order that the imported drugs be turned over to the FDA because some states were not parties to the suit originally brought by death row inmates in three states.
(“Appeals court says FDA can’t allow unapproved drug to be imported for executions,” Associated Press, July 23, 2013; Cook v. FDA, No. 12 – 5176, D.C. Cir. (2013)). See Lethal Injection.


